Texas AG investigating push to prescribe experimental puberty blockers not approved by FDA for kids
The Texas Attorney General’s office is investigating two pharmaceutical companies for their promotion of well-established drugs as puberty blockers despite the fact that they have not received approval from the Food and Drug Administration.
The Office of Texas’ Republican Attorney General Ken Paxton announced Monday that it was investigating Endo Pharmaceuticals and AbbVie Inc. under the Texas Deceptive Trade Practices Act.
“These pharmaceutical companies allegedly advertised and promoted hormone (puberty) blockers for unapproved uses without disclosing the potential risks associated with these drugs to children and their parents,” Paxton said in a statement.
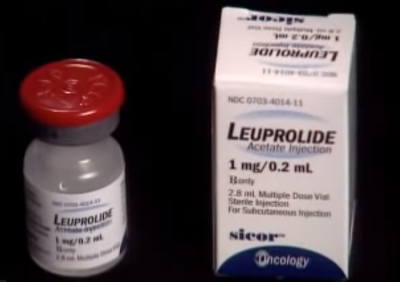
“Medications Supprelin LA and Lupron Depot are approved to treat children with Central Precocious Property (CPP), when the puberty process begins prematurely. And Vantas, along with other forms of Lupron, has been prescribed for palliative treatment of prostate cancer. These drugs are now being used to treat gender dysphoria even though they are not approved for such use by the Food and Drug Administration (FDA).”
The Attorney General’s Office cites the Texas Deceptive Trade Practices Act as the source of Paxton’s authority to investigate the pharmaceutical companies, explaining that the law gives the attorney general the power to “investigate false, misleading, and deceptive conduct by businesses in Texas.” In addition to sending out a tweet declaring that “I will not allow pharmaceutical companies to take advantage of #Texas children,” Paxton detailed some of the concerns pertaining to the experimental use of puberty blockers for children and teenagers.
I will not allow pharmaceutical companies to take advantage of #Texas children. I have officially opened an investigation. https://t.co/WCS5A0qiR5
— Attorney General Ken Paxton (@KenPaxtonTX) December 14, 2021
“The manufacture, sale, prescription, and use of puberty blockers on young teens and minors is dangerous and reckless,” Paxton said. “These drugs were approved for very different purposes and can have detrimental and even irreversible side effects.”
Earlier this year, the Karolinska University Hospital in Sweden elaborated on some of the “detrimental and even irreversible side effects” of puberty blockers when announcing that it would no longer prescribe the drugs for experimental use on children younger than 16 who are struggling with gender dysphoria. In a statement, the hospital warned that “these treatments are potentially fraught with extensive and irreversible adverse consequences such as cardiovascular disease, osteoporosis, infertility, increased cancer risk, and thrombosis.”
The American College of Pediatricians lists additional side effects of puberty blockers, which include “mood disorders, seizures, [and] cognitive impairment.”
In response to concerns about the side effects of puberty blockers, Arkansas became the first U.S. state to ban doctors from prescribing experimental puberty blockers, hormonal drugs and performing gender reassignment surgeries on children and teenagers with gender dysphoria by passing the Save Adolescents from Experimentation (SAFE) Act.
President Joe Biden's Department of Justice condemned the SAFE Act, filing a statement in an ACLU lawsuit against the Arkansas law declaring that “federal law bars the state of Arkansas from singling out [trans-identified] minors for specifically and discriminatorily denying their access to medically necessary care based solely on their sex assigned at birth.” The Biden administration contended that the law violated the Equal Protection Clause of the 14th Amendment to the U.S. Constitution.
A federal judge ultimately agreed with the Biden administration, striking down the SAFE Act shortly before it was set to take effect.
Shortly thereafter, Texas’ Republican Gov. Greg Abbott asked the state’s Department of Family and Protective Services to investigate whether prescribing puberty blockers to children with gender dysphoria or subjecting them to other experimental procedures constituted child abuse. The agency found that performing such procedures and prescribing such drugs to minors did constitute child abuse.
In a previous interview with The Christian Post, Dr. Paul Hruz of Washington University of Medicine in St. Louis, Missouri, expressed concern that using Lupron as a puberty blocker could affect children’s bone density. A study published earlier this year reached a similar conclusion, indicating that puberty blockers led to “reduced growth” in both height and bone strength among minors who took them.
Additionally, informed consent documents that a hospital in California asked children seeking to take puberty blockers and their parents state that “If your child starts puberty blockers in the earliest stages of puberty, and then goes on to gender-affirming hormones, they will not develop sperm or eggs. This means that they will not have biological children.”
A trans-identified doctor has also raised questions about the consequences of puberty blockers. Dr. Marci Bowers, who performed elective cosmetic surgeries on prominent trans-identified reality star Jazz Jennings, told Wall Street Journal contributor Abigail Shrier in an exclusive interview that “if you’ve never had an orgasm pre-surgery and then your puberty’s blocked, it’s very difficult to achieve that afterwards.”
“I worry about their reproductive rights later. I worry about their sexual health later and their ability to find intimacy,” Bowers added.
Ryan Foley is a reporter for The Christian Post. He can be reached at: ryan.foley@christianpost.com



























