White House dismisses pro-lifers' calls to fire FDA commissioner over abortion pill review
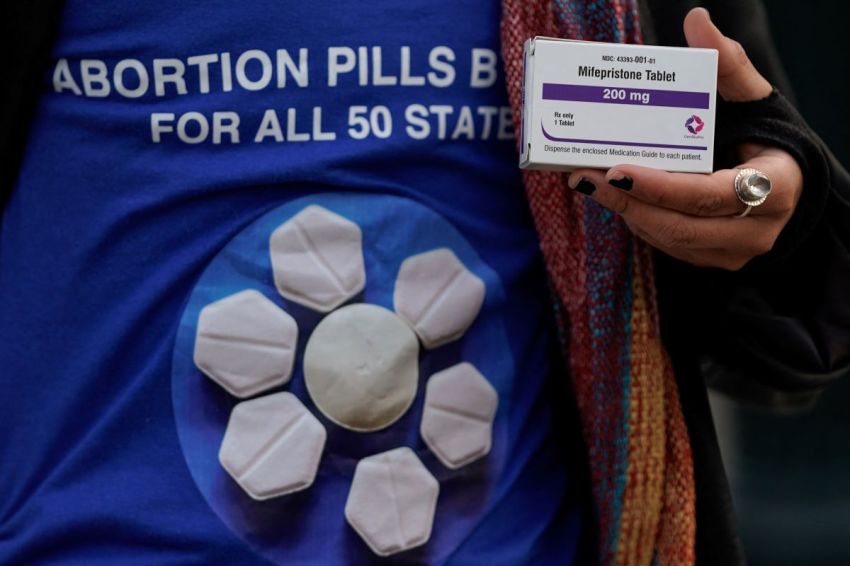
The White House says it has the "utmost confidence" in Food and Drug Administration Commissioner Marty Makary as pro-life leaders call for him to be fired following a report that he delayed a safety review of the abortion drug mifepristone until after the 2026 midterms.
Although Makary and Health and Human Services Secretary Robert F. Kennedy Jr. have promised lawmakers and state attorneys general that a safety review is underway, pro-life leaders expressed concern this week that the agency is stalling a safety review of the abortion drug following a Bloomberg report earlier this month that said Makary told agency officials to put it off until after the midterm elections.
Students for Life of America and Students for Life Action President Kristan Hawkins declared in a Tuesday statement that Makary's duty is to protect Americans' health, "not shield a political agenda."
"The FDA is stalling a crucial Chemical Abortion Pill safety study while women are harmed, abusers are empowered, and state laws are undermined," Hawkins wrote. "Makary, if you won't do the job, you're not the right person for it. Keep your promises and investigate these dangerous Chemical Abortion Pills NOW."
In response to the allegations, White House spokesperson Kush Desai said that Makary is "working diligently to ensure that Americans have the best possible, Gold Standard Science study of mifepristone."
"The White House maintains the utmost confidence in Commissioner Makary, whose leadership at the FDA has delivered and continues to deliver one landmark victory for the American people after another, from cracking down on artificial ingredients in our food supply to conducting the first safety review of baby formula in decades," Desai told The Christian Post.
"Uninformed attacks against Commissioner Makary from individuals outside the Administration will not change these facts," he added.
A White House official told The Hill in a statement this week that the study of mifepristone is not being "slow-walked," claiming that a comprehensive review like this one takes time.
During an interview with The Daily Signal, Makary said there are many rumors but insisted that the FDA is currently in the "data acquisition phase" of the review.
"We do an ongoing review, but we're also engaging in a robust study that can serve to validate or not validate other numbers that have been put out there in the literature," he said.
The grassroots anti-abortion group Susan B. Anthony Pro-Life America responded to Makary in a Wednesday tweet thread, calling on the FDA commissioner to reinstate the requirement that women visit a doctor in person before they can obtain abortion drugs.
The pro-life group also highlighted Makary's promise earlier this year about the FDA conducting a safety review, which he said was underway in September.
"Now, nearly a year into the Trump administration, @DrMakaryFDA's abortion drug safety review hasn't even passed the data-gathering phase, and there's no ETA. That's the definition of slow-walking," SBA Pro-Life America asserted.
In a statement, SBA Pro-Life America noted that in the months since Makary promised a safety review, multiple women have come forward and said their significant other forcibly gave them abortion drugs after obtaining them online.
Last month, the State Medical Board of Ohio suspended Dr. Hassan-James Abbas after he allegedly used his wife's information to order abortion drugs for his pregnant girlfriend. Abbas reportedly crushed the abortion drugs and held his girlfriend down as he attempted to force them into her mouth.
"Enough is enough: FDA Commissioner Makary should be fired immediately," SBA Pro-Life America President Marjorie Dannenfelser declared. "The FDA is doing nothing while every single day abortion drugs take the lives of children, put women and girls at serious risk, empower abusers and trample state pro-life laws."
"The FDA needs a new commissioner who will immediately reinstate in-person dispensing as it existed under President Trump's first term and immediately conduct a comprehensive study," Dannenfelser continued.
"Commissioner Makary is severely undermining President Trump and Vice President Vance's pro-life credentials and their position that states should have the right to enact and enforce pro-life protections," the pro-life leader added. "Makary must go."
According to a report published earlier this year by the Restoration of America Foundation, more than one in 10 women who use chemical abortion drugs experience an adverse event. The report also said that abortion drugs mailed to women are 22 times more dangerous than what the FDA discloses to patients.
The study, which used insurance claims from the years 2017 to 2023, identified over 600,000 patients who obtained 865,727 chemical abortions, which suggests repeat abortions.
"The dataset further shows an upward trend in serious adverse event rates in recent years," the study states. "In 2023 alone, 154,554 chemical abortions occurred with a serious adverse event rate of 11.2 percent."
Other studies have reportedly found that abortion drugs were associated with higher rates of severe or critical emergency room visits compared to surgical abortions and live births.
On Tuesday, the American Association of Pro-Life OBGYNs also condemned the FDA for approving a generic form of mifepristone when the agency promised to conduct a safety review.
"No more empty promises. Our physicians remain committed to caring for women facing severe complications caused by the lack of safeguards," the AAPLOG stated. "But the FDA must do its job — protecting women and girls not only from these dangerous drugs and from abusers who coerce and force their use."
Samantha Kamman is a reporter for The Christian Post. She can be reached at: samantha.kamman@christianpost.com. Follow her on Twitter: @Samantha_Kamman